FDA Adverse Events Summary for NUVARING* (Ethinyl estradiol; etonogestrel) between 2004 and 2012
Less than 10% of all adverse events are ever reported to the FDA
These charts and graphs provide data on adverse effects and events attributed to NUVARING. They are based on FDA Adverse Event reports received through the FDA Adverse Event Reporting System (FAERS) program.
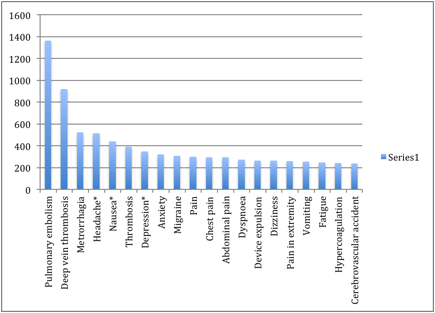
Top 20 Adverse Effects Associated with NUVARING
(reported in FDA Medwatch Reports)
| Side Effect | # of FDA Reports | |
| 1 | Pulmonary embolism | 1364 |
| 2 | Deep vein thrombosis | 921 |
| 3 | Metrorrhagia | 524 |
| 4 | Headache* | 514 |
| 5 | Nausea* | 438 |
| 6 | Thrombosis | 390 |
| 7 | Depression* | 349 |
| 8 | Anxiety | 320 |
| 9 | Migraine | 307 |
| 10 | Pain | 298 |
| 11 | Chest pain | 294 |
| 12 | Abdominal pain | 293 |
| 13 | Dyspnoea | 271 |
| 14 | Device expulsion | 265 |
| 15 | Dizziness | 262 |
| 16 | Pain in extremity | 259 |
| 17 | Vomiting | 257 |
| 18 | Fatigue | 246 |
| 19 | Hypercoagulation | 241 |
| 20 | Cerebrovascular accident | 239 |
* This side effect also appears in “Top 10 Side Effects of NUVARING ” in the drug’s Review Summary based on AskaPatient reviews.
(based on 39034 reports filed with the FDA between 2004 and 2012)
Adverse Event Reports are submitted by pharmaceutical companies (mandatory reporting is required by manufacturers, distributors, or importers), health care providers such as doctors (voluntary reporting), and by patients themselves (voluntary reporting). Voluntary reporting takes place under the FDA’s MedWatch program, where health care professionals and consumers submit reports to FDA when they find a problem with a drug, medical device, biologic, or other FDA-regulated product. An adverse event is any undesirable experience or extreme side effect associated with the use of a medical product. Adverse events include: death, life threatening event, hospitalization, disability or permanent damage, congenital anomaly or birth defect, medical product use requiring a surgical or other intervention, or other serious medical situation believed to be caused by the drug.
Source: https://www.askapatient.com/adverse-events.asp?drug=nuvaring (last accessed 4/9/2014