Source: https://decorrespondent.nl/1398/hoe-de-gezondheidsrisicos-van-de-nuvaring-uit-de-grafieken-verdwenen/108044013396-e5bdfbdd
Below is an investigative article that appeared in the Dutch on-line magazine De Correspondent. The original Dutch article can be read at the above link and a translation to English is given below. The translator included some comments or notes in brackets in the text.
[Translation]
The Dutch pharmaceutical company Organon left crucial data that pointed at increased health risks out of their research findings of birth control product Nuvaring. Also, the company did everything [possible] to minimize the risks of thrombosis in the ‘enclosed’ [= the pamphlet accompanying the product, I’ll use ‘label’ from here on, also shows up as ‘product insert’]. This shows up in the documents in possession of ‘De Correspondent’ [this blog]
From guest [blogger] Lucien Hordijk.
[http://en.wikipedia.org/wiki/Organon_International]
Correspondent Technologie & Surveillance
How health risks of the NuvaRing disappeared from the graphs
Maurits Martijn
Fall 2000. The American Federal Drug Administration had the task to judge a new birth control method of Dutch manufacture. The FDA had to judge if the NuvaRing, the revolutionary anti conception method by pharmaceutical company Organon [Brabant, province of NL, Oss, name of town] will be allowed access to the US market.
The NuvaRing is a plastic ring, applied vaginally as opposed to pills, secreting for three weeks a ‘stable’, ‘low’ dose of hormones. Easier than a daily tablet, at least as effective in preventing pregnancy and insertable by the women themselves. The most important advantage: the low quantity of hormones, keeping side effects to a minimum.
But precisely at the world’s greatest drug authority serious doubts arose about possible side effects of the NuvaRing. One of the medical researchers of the FDA, Daniel Davis, estimates that the number of thrombosis events in a few small-scale samples are twice as high as is known from pills with the same hormones already on the market. In comparison with anti-conception pills considered at that time as the safest, the chance [of thrombosis] is even four times as high.
It is clear, writes Davis in an internal evaluation that, if we approve this new product, we have to point out the higher risks than those associated [normally] with these [same] hormones in the scientific literature.
From documents in possession of ‘The Correspondent’, it appears that in the background a robust discussion has taken place between the FDA and Organon, that crucial data associated with increased risks of the product were left out of trial data and that the Company [Organon] successfully lobbied to keep the objections of the FDA out of the label.
Because if the label emphatically warns for an increased risk of thrombosis, entry into the market would have been dead on arrival. Doubts of the FDA caused turmoil at Organon, as the company from Oss at that point already had invested ten years in this anti-conception product, specifically to be safer and easier than the pills, used as comparison by the FDA for the ring. The pharmaceutical has all reasons to get a favorable indication [literally ‘manual’] and rigidly maintains their position in the negociations about the wording on the label.
The benefit of doubt
The FDA decides, after months of bickering, to overlook a few young victims of thrombosis during the drug trials. Instead of mentioning the doubts of the FDA about the hormones, the final label notes that it is unknown if the increased chances with pills regarding thrombosis are also valid for the NuvaRing.
The anti-conception ring is current used by 1.5 million women yearly.
Dutch and US employees of the ‘Regulatory Affairs’ division of Organon are satisfied when the hard fought business license is ‘pulled out of the fire’, documents show. The anti-conception ring, the first of its kind, is ready to conquer the world.
And a conquest it became. The anti-conception ring is used these days by 1.5 million women yearly and yielded MSD, the current manufacturer of the NuvaRing, 686 million dollar in 2013. According to MSD 179 million units were sold in the initial 10 years and it is meanwhile available in fifty countries. In the Netherlands the ring is provided 170 thousand times.
Lobbying to keep information ‘indoors’ [restricted]
But this commercial success has another side. Past June, the manufacturer concluded a lawsuit before the US high court [they use this term], where they were sued by thousands of users and next of kin because of the systematic withholding of information. Information that would point at health risks such as thrombosis, embolism and cerebral infarction. The result of the indictment is a settlement of $100 million, accepted by 95% of the approximately 3,800 plaintiffs. In return, MSD was found not guilty of withholding information that would inform users and prescribers of the ring of the higher risks of thrombosis and embolism as compared to other anti-conception products.
After a ‘muscled’ publication, ‘De Tegel-genomineerde publicatie van Anneke Stoffelen’ in the ‘Volkskrant’ [=a Dutch paper] of November last year about this lawsuit, a short discussion took place in the Netherlands about the NuvaRing. The Volkskrant article explored the doubling of the risk of blood clots of the NuvaRing compared to older anti-conception products. The ring would be just as risky as the Diane-35 pill, an anti-conception product that was withdrawn from the French market early 2013 because of the possibility that it caused the death of four young women. In the Netherlands, too, deaths were related to the use of the Diane-35 pill.
MSD, in a press release, ‘considered the article as information’ and ‘stands completely behind the NuvaRing’. Henk Jan Out, ex vice-president Women’s Health of the company, sneered in a column in ‘Artsennet.nl’ [link supplied] that the Volkskrant article was a mouthpiece of the US tort lawyers.
Caroline Doornebos, medical director at MSD, reacted during broadcasts of the NOS [=Dutch broadcast network] and Omroep Brabant ‘Het bericht’ van Omroep Brabant (local broadcaster) and assured ‘that there is no difference in risk of thrombosis from the NuvaRing as compared to other anti-conception products’.
From the court documents of the US lawsuit, witness declarations and correspondence with lawyers and scientists, another picture emerges. MSD has tried to prohibit public access to these documents but were overruled by the judge. [‘anders’ is a link to the judgement: ‘the decision in this matter’].
The Dutch enterprise Organon received before, during and after market entry different signals that the Nuvaring certainly entails an increased risk of bloodclots compared to other anti-conception products, but did not undertake anything to publicize this. Even worse [lit. ‘even stronger’], Dutch employees lobbied effectively to keep this information ‘indoors’ [restricted].
Onto the market
Before the NuvaRing was admitted to the market in the Netherlands as well in the US, a lot of discussions took place in the medical world about the hormones that would be used: ethinylestradiol, an estrogen, and etonogestrel, a so-called third generation progestogen.
Already in the nineties a lot of scientists agreed: anti-conception products with third and fourth generation progestogens have twice the chance of causing life-threatening side effects such as thrombosis compared to products with second generation progestogen. Out of sixteen trials thirteen confirmed this link. Three pharmaceutical industry sponsored studies found no link. That the manufacturer selected the patented etonogestrel for the NuvaRing is a logical choice business-wise: the hormone is a chemical derivative of its predecessor desogestrel, as of 1995 no longer patented. Still, etonogestrel was marked by the drug authorities as a ‘new molecular entity’.
For this reason, Organon hardly needed to supply new toxicological (animal and in-vitro) results. Also in the clinical phase of the research the company was allowed to refer to trials of the older products in the portfolio of Organon such as Marvelon and Mercilon.
In short, Organon was allowed to patent in an attractive way a new (sort of) molecule for their unique anti-conception product.
Sixteen women
To convince the drug authorities about the effectiveness and safety of the product, nine studies were performed by Organon.
These trials mostly took place in the Netherlands, with the participation of 2,586 women. The larger part of the studies investigated the effectiveness of the ring. One study was devoted to the way the hormones from NuvaRing were absorbed by the body. An essential study, since hormone levels that are too high are associated with a greater chance of side effects.
The study, that should validate the reason for existence of the product, consisted of sixteen participants and was performed by two scientists from Organon.
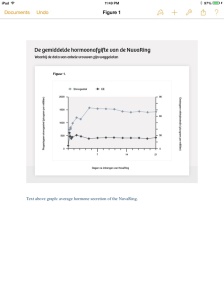
From the graphs and tables of this study, some of which also found their way onto the label, and in the publicized evaluation report of the ‘Dutch college for the evaluation of drugs’ [CBG], it shows that the average secretion of both hormones is stable. [lit.‘average release from both hormones with users stable happening’]. Good news for Organon.
Bron: Onderzoek 34.218 van Organon.
Text above graph: average hormone secretion of the NuvaRing.
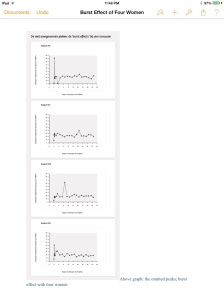
Whereby the data of a few women are omitted.
Bron: Expert Report of Shelley Ann Tischkau.
But the visualizations [graphs and tables] are not a complete representation of what was found with the participants [of the study].
From two research reports, introduced as evidence during the in June settled court case, it appears that the data of at least four women were omitted from the graphs and tables. These reports show that two medical employees of Organon confirm this. Therefore the US label, the publication of the study, the evaluation report of the CBG and the concluding document submitted to the FDA by Organon in order to obtain a license did not show a complete picture of the results of the study.
At four of the sixteen participants strong peaks of the hormone ethinylestradiol, a so called ‘burst effect’ took place. In two users the peak occurred a few hours after insertion of the ring, two other women experienced a surge of ethinylestradiol later on.
Source: Expert Report of Shelley Ann Tischkau
Above graph: the omitted peaks; burst effect with four women
Tom Sam, at the time a biochemist at Organon, admitted in a testimony during the court case that these four peaks were not processed into the graphs and tables. One of the researchers of the trial confirmed that the physicians prescribing the NuvaRing for women never would know that a few ‘burst effects’ took place.
The research data [lit. ‘little research’] that should show that the NuvaRing secretes hormones in an even manner, showed that the contrary was the case. In September 2001, when the FDA still had the application for NuvaRing under consideration, a worried Organon employee sent an e-mail to a colleague. It would be extremely damaging for the NuvaRing if the FDA were to be informed of the ‘burst effect’. He wrote: ‘Going to FDA to change these specifications is absolutely the LAST thing we should consider, i.e., that’s the worse[sic] possible scenario.’
Manipulated research
‘The Correspondent’ asked the Dutch drugs authority CBG if they were aware of the selective data use by the manufacturer in this specific research. The answer was that they could not possibly imagin that these data were omitted from the graphs. The authority [CBG] stated to get to the bottom of this now.
The CBG does emphasize that the alleged clinical advantage of the anti-conception ring is not mentioned in the Dutch label. ‘We have from the outset pointed to an increased risk of thrombosis, similar to Marvelon. We expressly prohibited Organon to claim in the pamphlet that the hormone levels were better of more even than the daily peaks that would be apparent with pills [lit. ‘that you would see with pills’].
In other words: the manipulated research by Organon is also taken by the Dutch authority as guidance but considered too skimpy [lit. ‘skinny’] to endorse the conclusions.
Manufacturer MSD, to this day, maintains the position that the ring has less side effects because of the low and even secretion of the hormones. In a reaction to an article in ‘De Volkskrant’ of November 2013, the company takes the position that ‘the hormones that prevent pregnancy are secreted evenly over a month. Even secretion means that no hormone peaks or valleys in the blood levels occur. Inasmuch that high levels can lead to side effects and low levels to insufficient effectiveness, striving for even blood levels is a very much accepted and logical starting point’.
Illustrating the point: on the MSD website ‘Anticonceptie.nl’ under the header ‘anticonceptiering’ [anti conception ring], ‘voordelen’ [advantages, link provided], ‘hier te lezen’ , read here. ‘An even secretion of hormones: the anti conception ring has therefore no daily hormone peaks like the pill’
The summary document from the CBG of 2001 endorses this position. There can be read, among other things, that the ring effectively prevents pregnancies but that less side effects in comparison with pills are insufficiently indicated: ‘Additionally, any clinical advantage of an absence of daily peak concentrations of estrogens and progestogens, in terms of a more favourable adverse event pattern, remains to be established.’
Thrombosis occurrences expunged from the label
Organon, during the registration procedure in the US, also did everything to get all unfavorable information out of the label.
In the clinical studies for market entry, three young women were shortly after application of the NuvaRing diagnosed with variations of thrombosis. On this basis, the FDA concluded that the risk of thrombosis of the NuvaRing was twice the risk of anti conception pills with Desogestrel, known to have a risk of twice the second generation pills.
‘We should really try to get it out of the text’
Susan Allen, with final responsibility of the FDA department judging studies, pointed specifically to one of these cases of thrombosis. A 26 year old woman in the clinical study developed a deep vein thrombosis in her left leg eight days after application of the NuvaRing.
It was for Allen a reason to order the manufacturer to emphasize in the pamphlet the increased risk of thrombosis of third and fourth generation progesterones, such as used in the ring. And more importantly, to specifically mention the thrombosis occurrence of the 26 year old. This way, physicians could judge for themselves if they wanted to prescribe a drug that was already associated with violent side effects in such a small research population.
From internal correspondence between marketing department and registration procedure department employees in the US and the Netherlands, it appeared that this proposal by the FDA was not well received [lit:‘did not really fall on fertile ground’]. Wim Mens, at the time working in the department of Regulatory Affairs in Oss, reported to his colleagues that he worried greatly about the mention of this case of thrombosis on the label. He wrote: ‘We should really try to get it out of the text’.
A marketing colleague in the US, David Stern, agreed. He suggested to use animal data from an earlier toxicological study as a trump card in the negotiations, to get the FDA to delete the ‘more important things’ such as the specific thrombosis case, deleted from the label. [this does not really make sense to me – but this is what it says here].
‘A good thing’
Surprisingly, Organon prevailed. By refusing the proposals of the drug authorities, the company managed to delay the initial registration procedure, the documents show. The 26 year old woman disappeared from the pamphlet and the position became ‘that it is unknown if the increased chances of thrombosis associated with third generation anti-conception products are also valid for vaginal application of etonogestrel’. The label contained only a general warning for blood clotting.
For Organon’s US marketing director this was still not enough, so he wrote to his colleagues: ‘The label change looks much better, however, I am still unhappy with the VTE section [Venous Thromboembolism, venous thrombus emboli, LH] of the label. Obviously the case that we presented to them [FDA, LH] has made some impact, in that they added the statement about being unknown if NuvaRing has this increased risk. What are the chances that this section can be removed all together?’
Retired team leader Willem de Boer, at the time team leader ‘Regulatory Affairs’ in Oss, was pleased by the finished label, which omitted the young woman with the deep artery thrombosis: ‘I have reviewed the new proposal for the NuvaRing Package Insert made by the FDA. To my satisfaction a number of critical issues have been implemented in the current proposal of the FDA (e.g. the deletion of the single VTE case).’
His US colleague Nancy Alexander also found the disappearance of the thromboses matter ‘a good thing’
An extra complaints department .
Still, Organon would not be able to prevent forever that their anti-conception ring would not increasingly be associated with increased thrombosis risk. Immediately after obtaining a trade license the company started new studies. Between the end of 2001 and begin 2004, again three more deep vein thrombosis cases occurred in a clinical setting.
Here, too, young women varying in age from 20 to 28 years were suffering side effects. In an interim report, generated by Dutch ex-employees, a VTE (Venous Thromboembolism) risk factor of 10.1 out of 10,000 women is calculated at that time. Five years after entering the market it appears after all that the anti-conception ring increases the risk for thrombosis.
Organon started the same year with a ‘VTE issue team’, tasked with the sensitive matter. Inside Organon the fear exists for underreporting, the phenomenon that the number of thrombosis victims is many times higher than estimated on the basis of calculations. Also, the public at large starts to get worried about the side effects, witness the dramatic increase in patient’s questions regarding the chances of a thrombosis induced by the ring, as mentioned by a US director.
Patients reporting side effects only will be acknowledged [lit. ‘called back’] after the judicial department has analyzed [lit. ‘x-rayed’] the report. Of all reported side effects for the complete drugs gamut of Organon, nine out of ten relate to the NuvaRing, according to an internal mail. Lacking capacity, an external entity is contracted to process the reports regarding the anti-conception ring.
Winning from the patch
The consternation of the users is, among other reasons, fueled by the action of the FDA a few months earlier. A direct competitor of the NuvaRing, the Evra anti conception patch manufactured by Johnson & Johnson, approved one month after the NuvaRing, gets end 2005 a review of the label and an infamous ‘black label box warning’. The patches, just like the rings, dispenses hormones in an alternative way and, according to the FDA, exposes women to a relatively high dosage of estrogen so consequently doubles the risk of dangerous side effects such as thrombosis.
The ‘black label warning’ has disastrous effects on the sale of the patches in the US. Where the company still sold about five million patches in 2005, in 2011 this decreased to less than 1.5 million. The NuvaRing sales skyrocket in 2005 by about 50% to 2.5 million units sold.
Organon is gets the chance to increase their sales at the expense of their main competitor but struggles internally with how to manage the matter of the thrombosis risk. Indeed, the patch touted the same stable hormone levels but still received an emphatic warning.
Convenient for the marketing
The pharmaceutical [Organon/MDA] obtains help from Susan Allen, the manager of the FDA who, five years earlier, wrestled with Organon about the thrombosis cases in the clinical studies. Under her leadership, the FDA not only approved the NuvaRing, but she also was instrumental in the Evra patch obtaining a license. Now she is making a living as an independent consultant for the pharmaceutical industry.
In 2006 Allen met Dutch director Hans Rekers, at that moment Vice President of Medical Affiars at Organon. They ‘sit at the table’ to craft a ‘fitting communications strategy’ now that the company is increasingly faced with ‘spontaneous reports of side effects like thromboses’.
The company decides to recruit the problems of their competitor by starting a marketing campaign touting the ‘advantages’ of the anti-conception ring over the maligned patch. A ‘dear doctor’ letter is crafted, in which the company informs physicians of the low estrogen exposure of the ring with respect to the patch. Also, a course for medical representatives is developed, training them to emphasize specifically the differences between the ring and the patch.
Never a word about the increased risk of thrombosis, as is apparent from the testimony of a US Sales Director, Sue Iannone: ‘It’s not something you’re going to see in a sales aid or in a users guide, because that’s not what healthcare providers typically want to talk about with representatives. And that’s not how we promote the products […] because they are safe if it’s approved by the FDA and there is a label.’
Women should be empowered to make a choice.
In a reaction of the company to questions of ‘The Correspondent’, MSD informs them [link supplied] ‘The questions of ‘The Correspondent and the answers of MSD’, that a substantial investigation is pending that will guarantee the safety of the product. The company points at the adaptations made worldwide to the ‘product inserts’, showing the results of both these researches that did not find a higher risk of thrombosis compared to second generation pills. [This is a really weird sentence: it mentions ‘pending investigation’, future tense, and than says the results of these pending investigations are known.]. However, one of these investigations according to a process document in the possession of ‘The Correspondent’ , does not have sufficient number of participants to give a decisive answer regarding the safety of the NuvaRing.
A well known Danish study under leadership of the Danish professor Øjvind Lidegaard with more than 3 million participants, found a NuvaRing thrombosis risk factor that was double that of second generation anti conception products. This study is not taken seriously by MSD on grounds of the methodology.
Lidegaard denounces the position of the manufacturer, which, according to him, has been trying for years to nip the discussion about the safety of the product in the bud: ‘There is strong scientific proof that the NuvaRing has at least twice the chances of causing thrombosis as compared to second generation pills’.
Asked if he feels that the drug authorities have to warn about risks, the professor answers affirmative. ‘As long as women are not informed about the differences in risk of thrombosis between the various products, they are not in the position to make an informed choice’.
Ironically, the manufacturer also considers adequate information for women fundamental. Only, the product insert of the NuvaRing does not mention, up to today, any study that flags the increased risks compared to the second generation pills.
This article is written by guest correspondent Lucien Hordijk